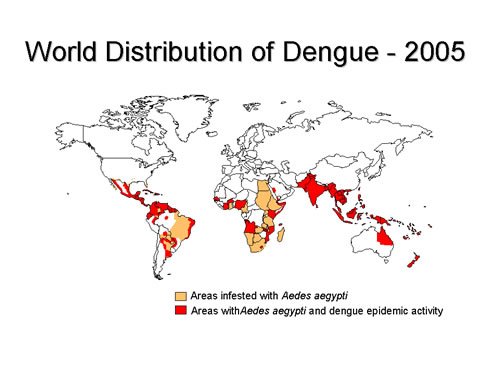
Fig.1. WHO stats on DENV distribution 2005
Dengue viruses cause what is known as a severe “flu-like” illness including symptoms such as rash, mild/high fever, headache and muscle pain. Although it is rarely fatal, in some instances it can develop into Dengue Haemorrhagic Fever (DHF), a potentially deadly complication. There are generally considered four sub-types (DENV-1 to 4) of Dengue virus due to the sufficient antigenic differences exhibited between them leading to little or no effective cross-neutralisation making it difficult to develop a ‘universal’ Dengue vaccine or treatment. The viruses are spread by certain Aedes species of mosquito passing infectious virus on during feeding by the females and due to the lack of treatments, the inhibition of mosquito activity has been seen as key to Dengue prevention.
A number of ‘vector control’ methods are being carried out to limit mosquito-human viral transmission which focus on disrupting the ecological niche in which the vector requires to breed – in this case stagnant water pools found in urban areas in which larva are found (e.g. rainwater filled tyres and cups). One major strategy is the biological control of Aedes populations including the use of mosquito larvae preying fish and invertebrates, release of Aedes infecting viruses or by genetic modification of mosquito genomes. An attractive means of control has come from the realisation that certain bacterial species (Wolbachia) - and only a specific strain of it - which naturally infects mosquitoes and other insects could inhibit dengue virus transmission thus negating the need for transgenic mosquitoes in the environment. These obligate, intracellular parasites which survive in the host cell’s cytoplasm are passed maternally from generation to generation. The bacteria can affect the mosquitoes and dengue virus in a number of ways, both general and specific for example: reduced lifespan of Aedes hosts; reduced dengue virus replication; increased antiviral immunity; and spatial exclusion of virus from the cytoplasm.
Fig.2. DENV transmission in Aedes species http://activity.ntsec.gov.tw/lifeworld/english/content/images/en_dis_c10.jpg
Frentiu et al report their investigations into the mechanism of reduced viral (dengue virus serogroup 2) replication at the cellular level paralleling earlier observations of whole organism Wolbachia infection. Their transition to a cell-culture experimental system may facilitate easier study of viral-host interactions and the group documents significantly reduced dengue virus replication in Wolbachia infected cell lines compared to non-infected controls possibly being related to an increased bacterial density within the cytoplasm. A bacterial ‘priming’ of the insect immune system may also contribute to decreased replication. They also show evidence that Wolbachia infected mosquitoes may display a fitness benefit compared to those not infected when challenged with dengue virus. This predicted increased fitness in the wild may aid the use of this biological control technique in a natural ecosystem. Measures such as these will benefit from the large, field trials already planned to study Aedes-Wolbachia interactions.
Fig.3. Wolbachia (Green) within a drosophila embryo imaged by confocal microscopy. http://www.genetics.org/content/vol178/issue4/cover.dtl
 Dengue virus is an important emerging arthropod-borne pathogen worldwide and is predicted to further increase its range into more temperate regions. Currently there is no effective vaccine or treatment and much research has focused on the interactions between virus and Aedes mosquito including the use of intracellular bacterial pathogens to limit viral replication and transmission. A number of strategies have been studied and seem attractive on a wider scale in endemic countries however the mechanisms of DENV/Wolbachia interactions and the effects on arthropods in the wild are understudied. Frentiu et al highlight the importance of a mechanistic understanding of dengue control and the development of novel control strategies.
Dengue virus is an important emerging arthropod-borne pathogen worldwide and is predicted to further increase its range into more temperate regions. Currently there is no effective vaccine or treatment and much research has focused on the interactions between virus and Aedes mosquito including the use of intracellular bacterial pathogens to limit viral replication and transmission. A number of strategies have been studied and seem attractive on a wider scale in endemic countries however the mechanisms of DENV/Wolbachia interactions and the effects on arthropods in the wild are understudied. Frentiu et al highlight the importance of a mechanistic understanding of dengue control and the development of novel control strategies.

No comments:
Post a Comment