The Paramyxoviruses Simian Virus 5 and Mumps Virus Recruit Host Cell CD46 To Evade Complement-Mediated Neutralization 
John B. Johnson,1 Ken Grant,2 and Griffith D. Parks1* Departments of Microbiology and Immunology,1 Pathology, Wake Forest University School of Medicine, Winston-Salem, North Carolina 27157-10642
Received 6 April 2009/ Accepted 12 May 2009
The complement system is a critical component of the innate immune response that all animal viruses must face during natural infections. Our previous results have shown that treatment of the paramyxovirus simian virus 5 (SV5) with human serum results in deposition of complement C3-derived polypeptides on virion particles. Here, we show that the virion-associated C3 component includes the inactive form iC3b, suggesting that SV5 may have mechanisms to evade the host complement system. Electron microscopy, gradient centrifugation, and Western blot analysis indicated that purified SV5 virions derived from human A549 cells contained CD46, a plasma membrane-expressed regulator of complement that acts as a cofactor for cleavage and inactivation of C3b into iC3b. In vitro cleavage assays with purified complement components showed that SV5 virions had C3b cofactor activity, resulting in specific factor I-mediated cleavage of C3b into inactive iC3b. SV5 particles generated in CHO cells, which do not express CD46, did not have cofactor activity. Conversely, virions derived from a CHO cell line that was engineered to overexpress human CD46 contained elevated levels of virion-associated CD46 and displayed enhanced C3b cofactor activity. In comparison with C3b, purified SV5 virions had very low cofactor activity against C4b, consistent with the known preference of CD46 for C3b versus C4b. Similar results were obtained for the closely related mumps virus (MuV), except that MuV particles derived from CHO-CD46 cells had higher C4b cofactor activity than SV5 virions. In neutralization assays with human serum, SV5 and MuV containing CD46 showed slower kinetics and more resistance to neutralization than SV5 and MuV that lacked CD46. Our results support a model in which the rubulaviruses SV5 and MuV incorporate cell surface complement inhibitors into progeny virions as a mechanism to limit complement-mediated neutralization.
We are constantly battling with viral invaders intent on using our cells as factories to build more invaders to enter the next person who is constantly at war with those viral… you get the picture. In order to thwart these attempts at assault we have evolved
multiple elaborate systems to protect ourselves. One such defense is the
complement cascade, an often overlooked but invaluable barrier to infection, which is a complex protein interaction network that altogether forms a major obstacle for would-be pathogens linking together key immune functions including microbial recognition, direct neutralisation and stimulation of cellular components. This ancient vertebrate defense is comprised of 30 separate soluble or membrane proteins which can react with each other and pathogens in order to mount an effective immune response and eliminate the source of infection. Unregulated activation of the complement cascade is damaging for host cells and so tight control is the best strategy to avoid aberrant cellular damage. Vertebrates carry out this function using a suite of regulators of complement activation or RCA proteins which limit the amount of activated components.
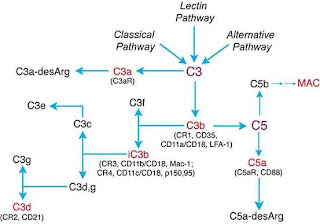
Fig. 1. Diagrammatic representation of the complement cascade showing the three alternative pathways of activation by virus particles (Classical, Lectin and Alternative) converging on the central C3 component essential for complement mediated neutralisation following cleavage into the C3a and C3b with C3b leading to downstream reactions such as viral opsonisation or coating, virolysis of free viral particles and localised inflammation. http://www.bioscience.org/2004/v9/af/1297/fig1.jpg
The downside to defense systems like these against generally
rapidly evolving viruses is that sooner or later the pathogens come up with effective strategies to bring down our barriers and infect our cells. Viruses have evolved myriad mechanisms to circumvent this system for example some large DNA viruses encode RCA protein mimics within their genome allowing viral controlled regulation of the complement cascade or retroviruses which direct the incorporation of host RCA proteins into their extracellular particles. However, little is known about how or even if RNA viruses (a large, diverse group of important human pathogens including the measles, ebola and influenza viruses) which have a limited genome size have any methods to get around this defense.
Johnson
et al report in the Journal of Virology the discovery and further investigation into the complement evasion strategies of Simian Virus 5 and
Mumps virus, two single-stranded, negative sense RNA viruses of the
Rubulavirus genus in the family
Paramyxoviridae. Following up on previous work that detected host CD46 protein (a membrane bound RCA glycoprotein that mediates C3b cleavage) in extracellular virus particles, the group show that CD46 and iC3b (the inactivated form of C3b, see above) localise to the SV5 and MuV envelope and that these virions can further promote inactivation of C3b to iC3b which appears to slow down neutralisation of viral infection (Fig.2). They take this as evidence of CD46 incorporation in virus particles leading to inactivation of extracellular complement thus delaying the host’s capacity to mount an effective defence via the complement cascade.

Fig.2. Electron microscopy and antibody staining of SV5 (left) and MuV (bottom right) virions for CD46. A neutralisation, plaque assay (top right) for MuV infecting cells expression CD46 (CHO-CD46) and those not (CHO) highlighting the decrease in neutralisation as measured by virus plaque formation when CD46 is present.
Their results demonstrate the importance of the complement cascade in virus/host interactions and pathogenesis and by showing that viral incorporation of host RCA proteins extends to a diverse and important group of human pathogens, highlights the need for the virus to avoid or limit host immune responses.



